Facile synthesis and magnetic phase transformation of Nd–Fe–B nanoclusters by oxygen bridging†
Abstract
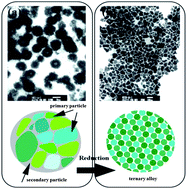
We demonstrate a facile chemical approach for fabricating Nd–Fe–B nanoclusters through oxygen bridging. The Nd–Fe–B nanoclusters comprised of small aggregated particles (∼2 nm diameter) could be successfully synthesized using

 Please wait while we load your content...
Please wait while we load your content...