Many whole cell-based assays in use today rely on flat, two-dimensional (2D) glass or plastic substrates that may not produce results characteristic of in vivo conditions. In this study, a three-dimensional (3D) cell-based assay scaffold was fabricated using a gas-in-foam templating technique. The scaffold was made of poly(vinyl alcohol), a water-soluble synthetic polymer with excellent film-forming, emulsifying, and biocompatible properties widely used in the biomedical field. The preliminary rheological studies on the solution of PVA and surfactant permitted us to disclose the significant physical parameters that influence the morphology of the ensuing materials. The scaffolds obtained were subjected to detailed analysis by light microscopy, Scanning Electron Microscopy (SEM), computed X-ray microtomography (μCT), infrared spectroscopy, and mechanical testing. Morphological investigations showed that the produced scaffolds are characterised by average void and interconnect diameters lying in the range of 200–300 and 30–150 μm, respectively, suitable for cell infiltration. Two different cross-linking procedures were adopted in order to modulate the mechanical properties of the PVA scaffolds. One made use of a bi-epoxide (PEGDGE), the other was based on glutaraldehyde (GA). The efficiency in terms of cross-linking density of the two procedures resulted in very different mechanical properties. Furthermore, in this article it is demonstrated how PVA foams can be processed into uniform, porous films suitable to be integrated with multi-well 2D culture plates in order to create a 3D analogue. The PEGDGE cross-linked scaffold was tested on C3A cells, a human hepatocyte cell line, representing an appropriate model for liver toxicity studies. Proliferation and cytotoxicity assays indicated good cell viability throughout the culture time, which was also confirmed by SEM analysis. Typical hepatic functions such as albumin and urea production and induction of Cyp3A4 enzyme activity following drug administration were satisfactory, thus proving the efficiency of this construct in maintaining specific liver functions.
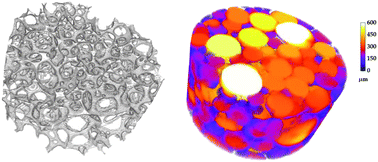

 Please wait while we load your content...
Please wait while we load your content...