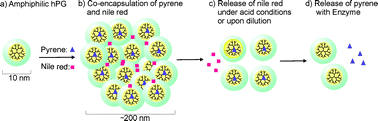
We here report on the synthesis of a bifunctional nanocarrier system based on amphiphilic hyperbranched polyglycerol (hPG), which is modified by introducing hydrophobic aromatic groups to the core and retaining hydrophilic groups in the shell. “Selective chemical differentiation” and chemo-enzymatic reaction strategies were used to synthesize this new core–shell type nanocarrier. The system shows an innovative bifunctional carrier capacity with both polymeric and unimolecular micelle-like transport properties. Hydrophobic guest molecules such as pyrene were encapsulated into the hydrophobic core of modified hPG via hydrophobic interactions as well as π–π stacking, analogous to a unimolecular micelle system. A second guest molecule, which has a high affinity to the shell like nile red, was solubilized in the outer shell of the host molecule, thus connecting the nanocarrier molecules to form aggregates. This model is confirmed by UV-Vis, fluorescence, atomic force microscopy, and dynamic light scattering, as well as release studies triggered by pH-changes and enzymes. Encapsulated guest molecules, respectively in the core and in the shell, present different controlled release profiles. The bifunctional nanocarrier system is a promising candidate for simultaneous delivery of different hydrophobic drugs for a combination therapy, e.g., in tumor treatment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...