Intrinsically radiolabeled multifunctional cerium oxide nanoparticles for in vivo studies†
Abstract
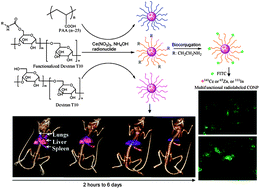
Cerium oxide nanoparticles (CONPs) have demonstrated protection properties against oxidation in various cells and tissues. The mechanism of this, however, is poorly understood. Monitoring the interaction of CONPs with biological compartments ‘in situ’ is crucial to understand their biochemical and physiological properties in vivo. In this paper, a multifunctional nanoparticle platform was obtained through an intrinsic radiolabeling strategy and extrinsic surface functionalization to combine dual imaging components (Single Photon Emission Computed Tomography/Optical Imaging, SPECT/OI) in one nanoparticle. The cell viability, cell uptake and overall in vivo biodistribution of CONPs were also manipulated through surface functionalization. The intrinsic radiolabeling strategy is demonstrated by incorporating radionuclides (141Ce, 111In or 65Zn) into CONPs and a radiolabeled CONP (rCONP) was coated with biocompatible polymers including Dextran T10 (DT10), poly(acrylic acid) (PAA), or functionalized DT10 (DT10-NH2, DT10-PEG and DT10-sulfobetaine). Fluorescent CONPs were obtained through conjugation of fluorescein isothiocyanate (FITC) with DT10-NH2 rCONP and used for cell imaging. The DT10 and DT10-NH2 rCONP did not show decreased viability up to 120 μg mL−1 whilst the PAA rCONP showed decreased viability beyond 40 μg mL−1. Variations in blood circulation and renal/hepatic clearance of rCONPs were demonstrated and were dependent on surface coating and the hydrodynamic size of nanoparticles. The ex vivo biodistribution results were reflected in SPECT imaging of 141Ce-rCONPs, showing accumulation in the liver and spleen of a living mouse over a one week period. The intrinsic radiolabeling and extrinsic surface modifications together determine the biophysical properties of CONPs and their potential applications for in vivo studies and biomedical imaging.

 Please wait while we load your content...
Please wait while we load your content...