Uniform epitaxial growth of Pt on Fe3O4 nanoparticles; synergetic enhancement to Pt activity for the oxygen reduction reaction†
Abstract
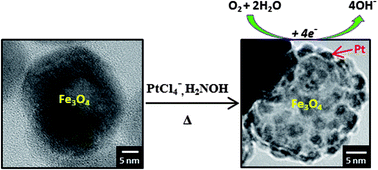
A synthetic strategy for achieving uniform shell-like epitaxial growth of Pt on Fe3O4 nanoparticles is introduced. The method involves the controlled high-density loading of Pt2+ by the linear growth of repeatedly stacked units of [Pt(NH2OH)4]2+ and [PtCl4]2−, followed by a subsequent reduction step. In comparison to commercial Pt-based fuel cell catalysts, the resulting Pt–Fe3O4 hybrid nanostructures were found to exhibit improved Pt specific activity for the electroreduction of oxygen in alkaline media, which is attributed to charge transfer from Fe3O4 to Pt. The Pt shell-type structure of the Pt–Fe3O4 hybrids was found to protect the Fe3O4 cores from corrosion, thus ensuring catalyst stability. A uniform Pt coating was also deposited evenly over SiO2 microspheres using this method, thus demonstrating its potential as a general strategy for platinum deposition on essentially any amine-functionalized surface.

 Please wait while we load your content...
Please wait while we load your content...