Synthesis and electrochemical performance of nanocrystalline Al0.4Mg0.2Sn0.4O1.6 and Al0.25Mg0.38Sn0.38O1.5 investigated by in situ XRD, 27Al/119Sn MAS NMR, 119Sn Mössbauer spectroscopy, and galvanostatic cycling
Abstract
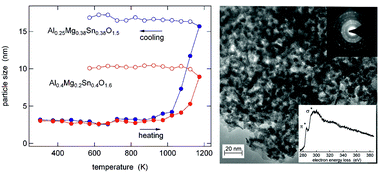
Nanocrystalline Al0.4Mg0.2Sn0.4O1.6 and Al0.25Mg0.38Sn0.38O1.5 were prepared by a co-precipitation method from Al(NO3)3·9H2O, MgSO4, and SnCl4·5H2O, followed by calcination at different temperatures. We performed in situ X-ray diffraction measurements at temperatures between 307 K and 1173 K and transmission electron microscopy. The results reveal a crystal structure equivalent to that of SnO2 cassiterite, very small crystallite sizes of about 3–4 nm, and high thermal stability. The local structure around Al and Sn was investigated by 27Al/119Sn NMR and 119Sn Mössbauer spectroscopy. These measurements show that the calcination results in the formation of [AlO4] and [AlO5] units, in addition to the initial [AlO6] environment, and in local disorder around the Sn atoms. The electrochemical performance was studied by galvanostatic cycling against Li metal. These experiments were performed on bare and carbon coated materials. Sn is the active component in these materials and undergoes an alloying reaction. Both Al0.4Mg0.2Sn0.4O1.6 and Al0.25Mg0.38Sn0.38O1.5 exhibit good electrochemical performance with a very stable cycling and a discharge capacity of 522 mA h g−1 and 385 mA h g−1, respectively, after 100 cycles. Their performance is strongly improved in comparison with pure SnO2 prepared by the same synthesis route.

 Please wait while we load your content...
Please wait while we load your content...