Synthesis, characterization and application of lanthanum-impregnated activated alumina for F removal†
Abstract
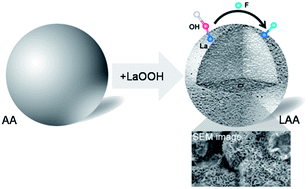
Elevated fluoride (F) in groundwater presents an obvious environmental concern. Adsorption using activated alumina (AA) is currently the best available technology for F removal, despite its low efficiency, pH dependence, and aluminium (Al) release. The motivation for our study is to synthesise a new F adsorbent by impregnating commercially available granulated AA with lanthanum oxide (LAA), and to explore its F adsorption mechanism on the molecular scale. Five cycles of lanthanum impregnation on AA followed by calcination at 573 K increased the La content up to 19.1% and the F removal from 18.1% by pristine AA to 92.4% by LAA. The SEM, TEM, XRD, TGA, and EXAFS results demonstrated that the 5–20 nm thin flakes of LaOOH on LAA were in an amorphous form, with 7.6 oxygen atoms around each La. This LaOOH layer was uniformly distributed inside the micropores of the 1–3 mm AA granules. LAA exhibited four fold higher F adsorption capacity than AA in the pH range 3.9 to 9.6, with substantially reduced Al release. The ability to regenerate and reuse LAA makes it an attractive sustainable material. Multiple complementary spectroscopic analyses demonstrated that ligand exchange between F and surface hydroxyl groups is the mechanism for F adsorption on LAA. Our work improves the understanding of F interaction with metal oxides on the molecular scale and presents an alternative solution for elevated F water treatment.

 Please wait while we load your content...
Please wait while we load your content...