Puzzling out the origin of the electrochemical activity of black P as a negative electrode material for lithium-ion batteries†
Abstract
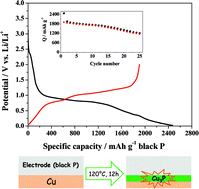
Black phosphorus prepared via the mineralization concept displays promising characteristics with respect to Li-ion battery applications. Although the theoretical specific capacity of black phosphorus as a negative electrode material is 2596 mA h g−1, a good cycling stability at high capacities, however, is still missing. Even worse, a large capacity drop after the first cycle is present and, depending on the electrode preparation, a poor first cycle coulombic efficiency (only 8%) represents a huge challenge to overcome. Herein we report on the performances of black phosphorus as a negative electrode material and display the roles of Cu3P and the copper current collector in this context. Furthermore, a composite material prepared by reacting pristine black phosphorus particles with a copper solution under solvothermal conditions shows a first cycle coulombic efficiency of 43%. We show that the presence of copper is crucial for the electrochemical performance of black phosphorus and that also the particle size and the electrode preparation procedure play a crucial role.

 Please wait while we load your content...
Please wait while we load your content...