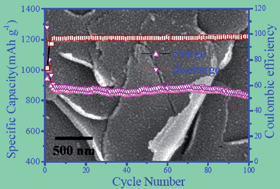
In this work, we have developed a new method to synthesize a Fe3O4@graphene (Fe3O4@GN) composite. First, the precursor was synthesized through the decomposition of ferric nitrate in the presence of graphene oxide in the mixed solvent of CO2–expanded ethanol. Then, the precursor was converted to the Fe3O4@GN composite via thermal treatment in N2 atmosphere. With the help of the CO2–expanded ethanol, Fe3O4 nanoparticles were coated on the surface of GN completely and uniformly with high loading. However, it is difficult to load Fe3O4 particles onto the surface of GN and most of the Fe3O4 particles were deviated away from GN and aggregated to form larger units in pure ethanol. When used as anode for Li-ion batteries (LIBs), the Fe3O4@GN composite with a graphene content of 25 wt% synthesized in CO2–expanded ethanol manifested excellent charge–discharge cycling stability and rate performance compared with the sample synthesized in ethanol. Such improved electrochemical performances should be attributed to the intimate contact between the GN and Fe3O4 nanoparticles in the composite. Since the present method does not need tedious pre-treatment, surfactant, or precipitate, it is a green or sustainable technology and the solvents could be recycled easily after simple phase separation. This facile method can be extended to the synthesis of other metal oxide composites, which are expected to have good performance as anode materials for LIBs and other applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...