Theoretical investigation of Li2MnSiO4 as a cathode material for Li-ion batteries: a DFT study†
Abstract
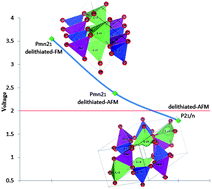
Transition metal lithium orthosilicates are of interest as cathode materials for lithium batteries. Density functional theory (DFT) calculations were performed to evaluate the structural and electrochemical properties of the different polymorphs of Li2MnSiO4. Our computational studies predict superior properties for the Pmn21 polymorph even after

 Please wait while we load your content...
Please wait while we load your content...