Flexible free-standing hollow Fe3O4/graphene hybrid films for lithium-ion batteries†
Abstract
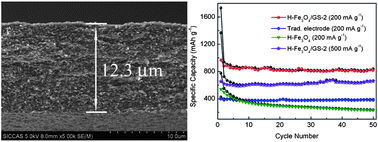
Flexible free-standing hollow Fe3O4/graphene (H-Fe3O4/GS) films were fabricated through vacuum filtration and thermal reduction processes, in which graphene formed a three-dimensional conductive network, with hollow and porous Fe3O4 spindles being captured and distributed homogeneously. Using the films as binder-free and free-standing electrodes for lithium-ion batteries, H-Fe3O4/GS with 39.6 wt% graphene exhibited a high specific capacity (1555 mA h g−1 at 100 mA g−1), enhanced rate capability and excellent cyclic stability (940 and 660 mA h g−1 at 200 and 500 mA g−1 after 50 cycles, respectively). The superior electrochemical performance of this novel material can be attributed to two factors. One is that the three dimensional (3D) graphene network formed is very helpful for keeping H-Fe3O4 in good electrical contact. Another is the short transport length for both lithium ions and electrons due to the porous nature which accommodates volume change and favors electrolyte penetration. It is believed that the strategy for preparing free-standing H-Fe3O4/GS films presented in this work will provide new insight into the design and synthesis of other metal oxide/GS electrodes for flexible energy storage devices.

 Please wait while we load your content...
Please wait while we load your content...