Programming nanoparticle aggregation kinetics with poly(MeO2MA-co-OEGMA) copolymers†
Abstract
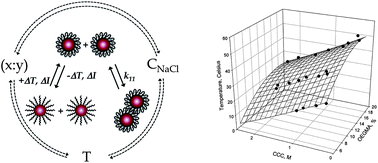
The ability to exert active control over the kinetic processes associated with dispersion stability is appealing for applications ranging from precision sensing to materials processing. Here, we demonstrate programmable aggregation dynamics using a system of gold nanoparticles sterically stabilized with a thermoresponsive copolymers consisting of di(ethylene glycol) methyl ether methacrylate (x = MeO2MA) and poly(ethylene glycol) methyl ether methacrylate (y = OEGMA). The copolymer ratio (x : y) dictates macromolecule hydrophilicity enabling control of the physicochemical properties of these nanoparticles through external stimuli such as temperature and ionic strength. A detailed characterization of these polymers and the variables used to control their physicochemical properties are presented. We determine the initial aggregation rate constants (k11), a widely adopted measure of nanoparticle stability, of Au@(MeO2MAx-co-OEGMAy) NPs as a function of co-polymer composition, ionic strength, and temperature. For NPs functionalized with a distinct ratio of copolymers (x : y), we observed the onset of measureable aggregation at especially high ionic strengths, normally >300 mM NaCl. While holding copolymer ratio constant, we observed a two order of magnitude increase in aggregation rate constants over less than 300 mM increase in NaCl concentration, 3.0 × 10−20 m3 s−1 < k11 < 2.0 × 10−18 m3 s−1. The results highlight the impact of the polymer structure and its interaction with the media on the swelling behavior and kinetic stability of core–shell thermoresponsive nanoparticles. We compare the inverse stability ratio, W−1, to a theoretical reversible association model in order to extract the underlying thermodynamics associated with interfacial hydration as a function of the co-polymer ratio and stimulus fields.

 Please wait while we load your content...
Please wait while we load your content...