Correlated vibrations in ion-pair dynamics in mechanoactivation identify functional domains of force-dependent titin kinase†
Abstract
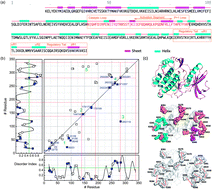
Titin kinase is a mechanoenzyme, whose activity is activated by mechanical stretching and binding of calcium sensors. Stretching causes local and global conformational changes of secondary structures and complex movements of ion pairs and transient formations of salt bridges. This paper applies the adaptive time series analysis approach to study the mechanical responses of ion-pair movements to stretch unfolding through steered molecular dynamics (SMD) simulations, focusing on the ion-pair dynamics of mechanoactivation obtained from the SMD trajectories. Temporal correlation analysis of the ion-pair time series shows that the activation process involves changes of secondary structure. Spectral analysis defined several groups and subgroups of the ion pairs with vibrational damping/resonance in the scale of ∼0.5 Å, corresponding to vibrational modes of chemical bonds. Examination of these groups revealed the locations or neighboring structures of the autoinhibitory loop, ATP binding cleft, catalytic loop, and P+1 loop, all key functional domains of this kinase. We propose that the correlated vibrations of subgroups of ion pairs have significant correlations with functional domains, which can be used to identify, a priori, special functional and structural features of folded proteins.

 Please wait while we load your content...
Please wait while we load your content...