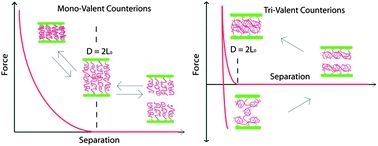
This paper describes the structure and properties of end-tethered anionic polyelectrolyte brushes containing counterions of tri-valent ruthenium hexamine (Ru(NH3)63+) and mono-valent sodium (Na+). Detailed studies of these planar brushes composed of the strong polyelectrolyte, poly(sodium styrenesulfonate), were performed via electrochemical experiments using cyclic voltametry (CV) and surface force experiments using the Surface Forces Apparatus (SFA). A comparison between electrochemical and force measurements provided physical information regarding polyelectrolyte brush height and the population of tri-valent Ru(NH3)63+ counterions inside a brush. Polyelectrolyte brushes were observed to transform from extended brushes dominated by the presence of mono-valent counterions, to collapsed brushes saturated with tri-valent counterions. This transformation occurred as a result of Ru(NH3)63+ replacement of Na+ counterions in the polyelectrolyte brushes. This Ru(NH3)63+ uptake was seen to be limited by either the diffusion of tri-valent ions in low ionic strength solutions or the equilibrium between mono and tri-valent ions at higher fixed ionic strengths. The collapsed state, seen only with brushes dominated by multi-valent counterions, also corresponded to an observed adhesive state between brushes. Adhesion was never seen with exclusively mono-valent ions present in solution. Ru(NH3)63+ was observed to be released from saturated brushes when placed in a purely mono-valent salt solution of sufficiently high ionic strength. All SFA and CV experiments were performed in solutions of three separate fixed ionic strengths, with brush collapse/saturation occurring more sudden and producing higher adhesion at lower total ionic strength.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...