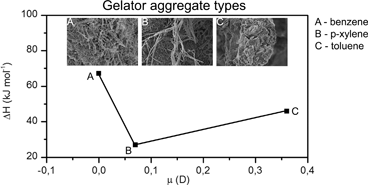
In this work, we report the complementary studies of supramolecular organogels composed of a newly synthesized low molecular mass gelator 4-(4-morpholinyl)-3-nitro-benzoylhydrazide (1) with benzene, toluene, and p-xylene. Intermolecular hydrogen bonding and π–π stacking interactions are the main driving forces promoting gelation of the system and the self-assembly of the new gelator molecules. The former interactions were revealed by the FT-IR and Raman studies, whereas the latter ones were postulated on the basis of the molecular structure of the gelator, UV-Vis spectra and comparison with the previously published data for other hydrazide derivatives. The strengths of the hydrogen bonding interactions are comparable as indicated by the FT-IR spectra analysis. Therefore, we correlated the differences in the calculated enthalpies of the gelator aggregates of 1 in the gels studied with the differences in the strengths of the π–π stacking interactions. The gel of 1 with benzene is characterized by the highest value of enthalpy. The images taken by the SEM and POM methods reveal differences in the architecture of gelator 1 aggregates, which are lamellar-like in toluene and fibrillar in benzene and p-xylene. The dispersion of spin–lattice relaxation times of solvents in the gel phase, observed by the NMR relaxometry method at low frequencies, is an indicator of the solvent–gelator interactions. As a result of this interaction, a significant slowing down of the motion (5 orders of magnitude as compared to bulk solvents) of the fraction of solvent molecules at the pore surface in the gel phase takes place. The diffusion of solvents in gels is restricted as shown by PGSE NMR. The apparent diffusion coefficient measured as a function of diffusion time can be used to estimate the pore size of the gel matrix, which for the gel of 1 with toluene is about 22 μm.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...