Cationic poly(2-oxazoline) hydrogels for reversible DNA binding
Abstract
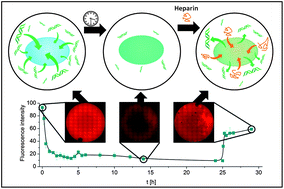
A new 2-oxazoline monomer with a Boc-protected amino group in the side chain (BocOx) was synthesized. Homopolymerization as well as copolymerization with 2-ethyl-2-oxazoline (EtOx) revealed a pseudo first order kinetic. A series of homopolymers was synthesized, deprotected and characterized regarding their structure and thermal properties. The copolymerization with EtOx yielded a series of water soluble polymers with varying amino contents. After deprotection it was shown by the ethidium bromide assay that these polymers were able to form complexes with DNA. Treatment with epichlorohydrin leads to the formation of hydrogels. The swelling properties of the gels were investigated and it could be demonstrated that also the polymeric scaffolds were able to immobilize DNA from aqueous solution. Furthermore, the release of the DNA was accomplished using heparin.

 Please wait while we load your content...
Please wait while we load your content...