Theory and simulations of crystalline control via salinity and pH in ionizable membranes
Abstract
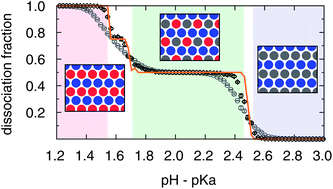
Many amphiphilic molecules with ionizable charged groups self-assemble into membranes. Their degree of ionization is a function of the pH and salt concentration in the solution, and it can also be suppressed or enhanced depending on the local crystalline structure on the membrane, which must be computed self-consisitently via the competition of short range and electrostatic interactions. We consider here a dense two-dimensional binary mixture consisting of positive and negative species of equal concentration. Each particle may be either charged according to its type or neutral, specifying its ionization state. We analyze this co-assembled system by numerically exact optimization and by continuum Monte Carlo simulations including short range and electrostatic interactions among all the particles. We find the optimal structure to be a triangular lattice for high salt concentrations, a face-centered rectangular lattice for intermediate salt concentrations, and a square lattice for low salt concentrations. At neutral pH, this crossover occurs gradually over a wide range of salt concentrations, while for highly acidic or basic solutions, it is much more abrupt. At intermediate values of pH, the unit cells become more complicated, causing the dissociation curve to follow a staircase function.

 Please wait while we load your content...
Please wait while we load your content...