Formation of sub-valent carbenoid ligands by metal-mediated dehydrogenation chemistry: coordination and activation of H2Ga{(NDippCMe)2CH}†
Abstract
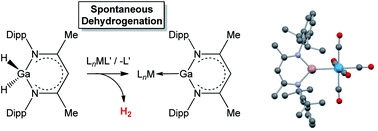
Reactions of the β-diketiminato (‘Nacnac’) stabilized gallium dihydride H2Ga{(NDippCMe)2CH} with a range of mono- and dinuclear metal carbonyl reagents are characterized by loss of dihydrogen and formation of donor/acceptor complexes featuring the Ga(I) carbenoid ligand : Ga{(NDippCMe)2CH}. Thus, far from simply mimicking the chemistry of the corresponding alane H2Al{(NDippCMe)2CH}, which yields κ1 and κ2 Al–H σ-complexes with similar reagents, the weaker nature of Ga–H bonds leads to extensive bond activation chemistry and enables an unprecedented dehydrogenative route to Ga(I) ligand systems. By consideration of the chemistry of dinuclear systems, two alternative pathways are revealed for this chemistry, with either H2 or M–H bonds acting as the ultimate hydrogen sink.

 Please wait while we load your content...
Please wait while we load your content...