Direct monofluoromethylation of O-, S-, N-, and P-nucleophiles with PhSO(NTs)CH2F: the accelerating effect of α-fluorine substitution†
Abstract
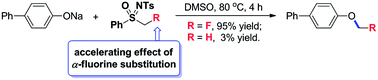
An efficient and direct monofluoromethylation of O-, S-, N-, and P-nucleophiles with PhSO(NTs)CH2F 1 has been developed. In contrast to the previously known detrimental effect of α-fluorine substitution on SN2 reactions, the current monofluoromethylation is accelerated by the α-fluorine substitution. Based on a mechanistic study, a new reactivity of sulfoximine (as a radical monofluoromethylation reagent) is disclosed.

 Please wait while we load your content...
Please wait while we load your content...