The Ugi four-component reaction enables expedient synthesis and comparison of photoaffinity probes†
Abstract
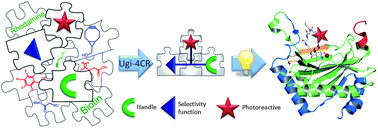
Photoaffinity probes are increasingly being used for the study of biological interactions; however, the lack of structure–activity relationship studies has hindered their rational application. We describe the use of the Ugi four-component reaction (U-4CR) for the expedient and versatile

 Please wait while we load your content...
Please wait while we load your content...