Rh(iii)-catalyzed C–H activation/cycloaddition of benzamides and methylenecyclopropanes: divergence in ring formation†
Abstract
An unprecedented Rh(III)-catalyzed C–H activation/
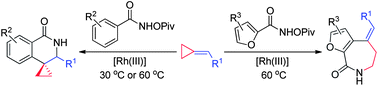
- This article is part of the themed collection: C-H Functionalization

 Please wait while we load your content...
Please wait while we load your content...