Iridium(iii)-bis(oxazolinyl)phenyl catalysts for enantioselective C–H functionalization†
Abstract
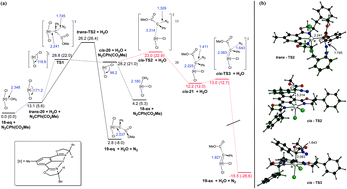
Recently, a small number of diverse iridium complexes have been shown to catalyze unusual atom transfer C–H functionalization reactions. To further our understanding and enhance the utility of iridium complexes for C–H functionalization, we report the design and synthesis of a family of iridium(III)-bis(oxazolinyl)phenyl complexes. The ability to tune the
- This article is part of the themed collection: C-H Functionalization

 Please wait while we load your content...
Please wait while we load your content...