Synthesis and electronic structure of a two dimensional π-conjugated polythiophene†
Abstract
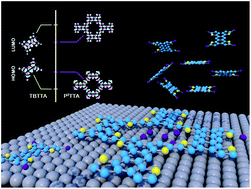
We report the synthesis and first electronic characterization of an atomically thin two dimensional π-
* Corresponding authors
a
Institut National de la Recherche Scientifique – Énergie, Matériaux et Télécommunications and Centre for Self-Assembled Chemical Structures, Université du Québec, Varennes, QC J3X 1S2, Canada
E-mail:
cardenas@emt.inrs.ca, r.gutzler@fkf.mpg.de, rosei@emt.inrs.ca
b
Department of Chemistry and Centre for Self-Assembled Chemical Structures, McGill University, Montreal, QC H3A 2K6, Canada
E-mail:
dmitrii.perepichka@mcgill.ca
c Fyzikální ústav AV ČR, v.v.i., Praha 8, Czech Republic
d
Sincrotrone Trieste S.C.p.A., Basovizza-Trieste, Italy
E-mail:
vondrac@fzu.cz
e
Institut Jean Lamour, Nancy-Université, lès-Nancy, France
E-mail:
yannick.fagot@univ-lorraine.fr, daniel.malterre@ijl.nancy-universite.fr
We report the synthesis and first electronic characterization of an atomically thin two dimensional π-

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
L. Cardenas, R. Gutzler, J. Lipton-Duffin, C. Fu, J. L. Brusso, L. E. Dinca, M. Vondráček, Y. Fagot-Revurat, D. Malterre, F. Rosei and D. F. Perepichka, Chem. Sci., 2013, 4, 3263 DOI: 10.1039/C3SC50800E
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence. You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
