Electrochemical water splitting by gold: evidence for an oxide decomposition mechanism†
Abstract
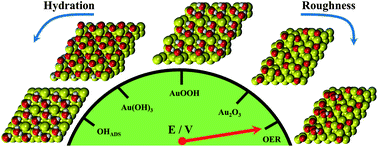
In this paper we study through a multiplicity of experimental and theoretical techniques the electrochemical evolution of oxygen on gold, the metal on which water splitting was initially discovered more than two centuries ago. The evidence obtained with a combination of in situ surface-enhanced Raman spectroscopy, online electrochemical mass spectrometry and density functional theory calculations suggests the existence of several mechanisms for the evolution of O2 on Au electrodes, depending on the electrode potential. Significantly, at approximately 2.0 V vs. RHE the first O2 that is evolved consists of two oxygens from the surface oxide, suggesting an oxide decomposition or oxide disproportionation step. At somewhat higher potentials, O2 is formed by a combination of oxygen from the oxide lattice and oxygen provided by water. The oxide decomposition step implies a more three-dimensional mechanism for oxygen evolution than suggested in previous mechanisms, which involve only surface-adsorbed intermediates.

 Please wait while we load your content...
Please wait while we load your content...