Understanding the competitive dehydroalkoxylation and dehydrogenation of ethers with Ti–C multiple bonds†
Abstract
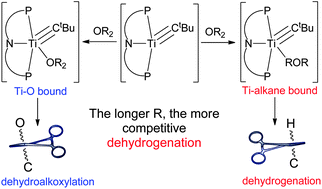
The divergent reactivity of a transient titanium neopentylidyne, (PNP)Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu (A) (PNP = N[2-PiPr2-4-methylphenyl]2−), that exhibits competing
CtBu (A) (PNP = N[2-PiPr2-4-methylphenyl]2−), that exhibits competing ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHtBu(CH2tBu), show pseudo first-order decay rates on titanium (kavg = 6.2 ± 0.3 × 10−5 s−1, at 29.5 ± 0.1 °C, overall), regardless of the substrate or reaction pathway that ensues. Also, no significant kinetic isotope effect (kH/kD ∼ 1.1) was found between the activations of Et2O and Et2O-d10, in accord with
CHtBu(CH2tBu), show pseudo first-order decay rates on titanium (kavg = 6.2 ± 0.3 × 10−5 s−1, at 29.5 ± 0.1 °C, overall), regardless of the substrate or reaction pathway that ensues. Also, no significant kinetic isotope effect (kH/kD ∼ 1.1) was found between the activations of Et2O and Et2O-d10, in accord with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHtBu(CH2tBu) with a t1/2 = 3.2 ± 0.4 h, across all
CHtBu(CH2tBu) with a t1/2 = 3.2 ± 0.4 h, across all ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHtBu(OR) (R = Me, Et, nPr, nBu, iPr, tBu) formed from dehydroalkoxylation reactions were also independently prepared by salt metatheses, and extensive NMR characterization of these products is provided. Finally, combining theory and experiment we discuss how each reaction pathway can be altered and how the binding event of
CHtBu(OR) (R = Me, Et, nPr, nBu, iPr, tBu) formed from dehydroalkoxylation reactions were also independently prepared by salt metatheses, and extensive NMR characterization of these products is provided. Finally, combining theory and experiment we discuss how each reaction pathway can be altered and how the binding event of

 Please wait while we load your content...
Please wait while we load your content...