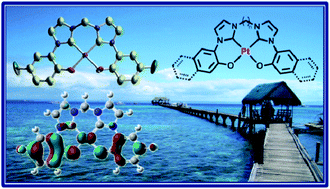
The synthesis, structures and photophysical properties of the charge-neutral Pt(II) complexes (1–6) and their Pd(II) (7) and Ni(II) (8) congeners supported by tetradentate dianionic bis[phenolate-(N-heterocyclic carbene)] ligands are described. The X-ray crystal structures of two solvatomorphs of 2, which has p-F substituents on the tetradentate ligand, have been determined. The photophysical properties of all the complexes were examined. In THF solutions, 1–4 display deep blue phosphorescence (λmax = ∼440–460 nm, Φe = 3–18% and τ = 0.5–3.5 μs). In solutions at room temperature, 5–8 show profoundly different luminescence properties from being virtually non-emissive (Φe < 10−3) for 6–8 to highly emissive (Φe = 15%) with much red-shifted phosphorescence (λmax = ∼530 nm) and a long emission lifetime (τ = 47.2 μs) in the case of 5. Time-dependent density functional theory (TDDFT) calculations reveal that the tetradentate bis(phenolate-NHC) ligands in 1–4 provide a rigid scaffold for preserving a tightly bound Pt(II) in a square-planar coordination geometry in the T1 as in the S0 states and the blue emission is derived from the T1 state having predominant ligand (πAr–O)-to-ligand (π*NHC) charge transfer (LLCT) character. A switch of orbital parentage from LLCT to ligand-centred (LC) π–π* is responsible for the long emission lifetime and vibronically structured emission displayed by 5 when compared to that of 1–4 and 6. Both femtosecond time-resolved fluorescence (fs-TRF) and nanosecond time-resolved emission (ns-TRE) measurements were conducted on 2 and 4 to directly probe the excited-state dynamics after photoexcitation. Excellent thermal stability of the fluorine-free complex 4 and its higher emission quantum yield (relative to 1 and 3), and using 9-(4-tert-butylphenyl)-3,6-bis(triphenylsilyl)-9H-carbazole (CzSi) as host material, led to the fabrication of highly efficient deep blue OLEDs with peak current efficiency of 24 cd A−1 and white organic light-emitting devices (WOLEDs) with peak current efficiency of 88 cd A−1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...