Complex [tBuOCO]W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(tBu)(THF)2 (1) {where tBuOCO = [2,6-(tBuC6H3O)2C6H3]3−, THF = tetrahydrofuran} polymerizes acetylenes (R-phenylacetylene (R = H, p-OMe, p-F, 3,5-diCF3), 1-decyne, 3,3-dimethyl-1-butyne, and trimethylsilylacetylene) to form π-conjugating polymers. Upon treating 1 with 2 equiv. of phenylacetylene in toluene-d8 at −35 °C, two isolable products form. These two products are [O2C(tBuC
C(tBu)(THF)2 (1) {where tBuOCO = [2,6-(tBuC6H3O)2C6H3]3−, THF = tetrahydrofuran} polymerizes acetylenes (R-phenylacetylene (R = H, p-OMe, p-F, 3,5-diCF3), 1-decyne, 3,3-dimethyl-1-butyne, and trimethylsilylacetylene) to form π-conjugating polymers. Upon treating 1 with 2 equiv. of phenylacetylene in toluene-d8 at −35 °C, two isolable products form. These two products are [O2C(tBuC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )W(η2-HC
)W(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)] (2-tttBu) and [O2C(PhC
CPh)] (2-tttBu) and [O2C(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )W(η2-HC
)W(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)] (2-Ph) {where OC(tBuC
CtBu)] (2-Ph) {where OC(tBuC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )O = [2,6-(tBuC6H3O)2C6H3(tBuC
)O = [2,6-(tBuC6H3O)2C6H3(tBuC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )]4−, OC(PhC
)]4−, OC(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )O = [2,6-(tBuC6H3O)2C6H3(PhC
)O = [2,6-(tBuC6H3O)2C6H3(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )]4−} and derived from an apparent reductive alkylidyne migratory insertion into a metal–arene bond. Complexes 2-tttBu and 2-Ph polymerize acetylene and a wide variety of monosubstituted acetylenes including phenylacetylene derivatives, 1-decyne, 3,3-dimethyl-1-butyne and trimethylsilylacetylene. With a substrate to catalyst loading ratio of 25 000 : 1, complex 2-tttBu polymerizes phenylacetylene with a turnover number (TON) of 17 233. Additionally, 2-tttBu polymerizes phenylacetylene and 1-decyne with catalytic activities up to 5.64 × 106 gPPA mol−1 h−1 and 7.98 × 106 gPA mol−1 h−1, respectively. 2-tttBu also polymerizes the disubstituted acetylene, 1-phenyl-1-propyne. NMR spectroscopic and single crystal X-ray structural studies provide compelling evidence for polymer chain growth via an insertion ring-expansion mechanism.
)]4−} and derived from an apparent reductive alkylidyne migratory insertion into a metal–arene bond. Complexes 2-tttBu and 2-Ph polymerize acetylene and a wide variety of monosubstituted acetylenes including phenylacetylene derivatives, 1-decyne, 3,3-dimethyl-1-butyne and trimethylsilylacetylene. With a substrate to catalyst loading ratio of 25 000 : 1, complex 2-tttBu polymerizes phenylacetylene with a turnover number (TON) of 17 233. Additionally, 2-tttBu polymerizes phenylacetylene and 1-decyne with catalytic activities up to 5.64 × 106 gPPA mol−1 h−1 and 7.98 × 106 gPA mol−1 h−1, respectively. 2-tttBu also polymerizes the disubstituted acetylene, 1-phenyl-1-propyne. NMR spectroscopic and single crystal X-ray structural studies provide compelling evidence for polymer chain growth via an insertion ring-expansion mechanism.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(tBu)(THF)2 (1) {where tBuOCO = [2,6-(tBuC6H3O)2C6H3]3−, THF =
C(tBu)(THF)2 (1) {where tBuOCO = [2,6-(tBuC6H3O)2C6H3]3−, THF = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )W(η2-HC
)W(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)] (2-tttBu) and [O2C(PhC
CPh)] (2-tttBu) and [O2C(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )W(η2-HC
)W(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)] (2-Ph) {where OC(tBuC
CtBu)] (2-Ph) {where OC(tBuC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )O = [2,6-(tBuC6H3O)2C6H3(tBuC
)O = [2,6-(tBuC6H3O)2C6H3(tBuC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )]4−, OC(PhC
)]4−, OC(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )O = [2,6-(tBuC6H3O)2C6H3(PhC
)O = [2,6-(tBuC6H3O)2C6H3(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )]4−} and derived from an apparent reductive alkylidyne migratory insertion into a metal–
)]4−} and derived from an apparent reductive alkylidyne migratory insertion into a metal–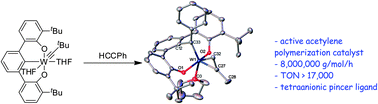

 Please wait while we load your content...
Please wait while we load your content...