On the reactivity and selectivity of donor glycosides in glycochemistry and glycobiology: trapped covalent intermediates
Abstract
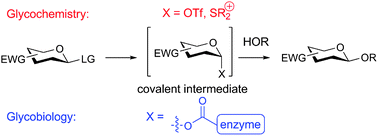
The reactivity of sugar donors and the stability of covalent intermediates formed in both chemical and biological systems is an active subject of study in both glycochemistry and glycobiology. Knowledge of the structure of these intermediates is vital for understanding reactivity and stereoselectivity in glycosidic bond formation, and in glycosidic bond destruction in the case of enzymatic
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...