On-surface cross-coupling methods for the construction of modified electrode assemblies with tailored morphologies†‡
Abstract
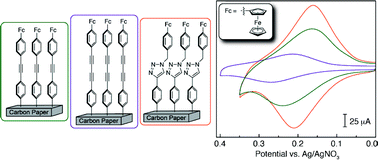
Controlling the molecular topology of electrode–catalyst interfaces is a critical factor in engineering devices with specific electron transport kinetics and catalytic efficiencies. As such, the development of rational methods for the modular construction of tailorable electrode surfaces with robust molecular wires (MWs) exhibiting well-defined molecular topologies, conductivities and morphologies is critical to the evolution and implementation of electrochemical arrays for sensing and catalysis. In response to this need, we have established modular on-surface Sonogashira and Glaser cross-coupling processes to synthetically install arrays of ferrocene-capped MWs onto electrochemically functionalized surfaces. These methods are of comparable convenience and efficiency to more commonly employed Huisgen methods. Furthermore, unlike the Huisgen reaction, this new surface functionalization chemistry generates modified electrodes that do not contain unwanted ancillary metal binding sites, while allowing the bridge between the ferrocenyl moiety and electrode surface to be synthetically tailored. Electrochemical and surface analytical characterization of these platforms demonstrate that the linker topology and connectivity influences the ferrocene redox potential and the kinetics of charge transport at the interface.

 Please wait while we load your content...
Please wait while we load your content...