Synthesis of interesting β-nitrohydrazides through a thiourea organocatalysed aza-Michael addition†
Abstract
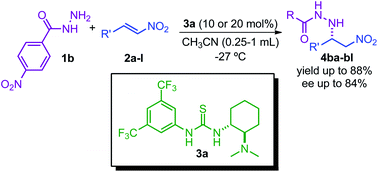
The synthesis of antimicrobial β-nitrohydrazides, as the target product of our reaction, is reached for the first time under organocatalytic enantioselective conditions. We accomplish this goal by using a thiourea organocatalysed aza-Michael addition reaction of hydrazide to nitroalkenes, furnishing final products with good yields and good enantiomeric ratios. The developed methodology is one of the rare examples of catalytic reactivity using hydrazide where the reaction proceeds through its N2 atom. Our developed strategy extends the generality of this area of research due to the difficulties of designing new nitrogen nucleophiles with high activity, and in addition, this is one of the scarce examples where nitroalkenes and chiral thioureas have been used in asymmetric organocatalytic aza-Michael addition reactions.

 Please wait while we load your content...
Please wait while we load your content...