Two-component gel of a D–π–A–π–D carbazole donor and a fullerene acceptor†
Abstract
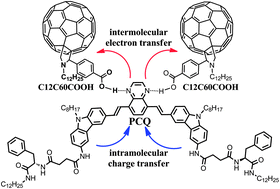
A D–π–A–π–D carbazole derivative (PCQ) with a quinoxaline moiety was designed and synthesized. Its photophysical properties in solution were studied. Moreover, PCQ was found to be a highly efficient gelator toward various apolar and polar organic solvents with the critical gelation concentrations (CGCs) as low as 0.06 wt/vol%. Spectral studies and molecular dynamic stimulation revealed that the intermolecular H-bonds and π–π stacking interactions might be responsible for guiding the self-assembly processes and the gel formation. Interestingly, PCQ could construct two-component gel with fullerene derivative driven by intermolecular hydrogen bonds. Moreover, the two-component gel film could generate photocurrent under light irradiation, indicating photo-induced electron transfer from the PCQ aggregate to the fullerene derivative.

 Please wait while we load your content...
Please wait while we load your content...