A highly efficient thiourea catalyzed dehydrative nucleophilic substitution reaction of 3-substituted oxindoles with xanthydrols†
Abstract
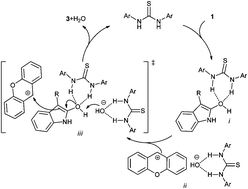
We report a highly efficient thiourea catalyzed dehydrative nucleophilic substitution reaction. The Schreiner's thiourea catalyst A1 catalyzed the alkylation of 3-substituted oxindoles with xanthydrols well, to furnish quaternary oxindoles in high yield. The ESI-MS analysis confirms the interaction of 3-substituted oxindole 1 with the thiourea, which might facilitate the oxindole–hydroxindole tautomerization for the alkylation.

 Please wait while we load your content...
Please wait while we load your content...