Adsorption of Acid red onto chitosan/rectorite composites from aqueous solution†
Abstract
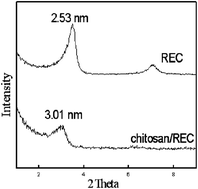
The chitosan/rectorite composites were prepared and characterized by FTIR and XRD. –NH2 and –OH groups of chitosan formed hydrogen bonds with the –OH groups in rectorite. The interlayer distance of rectorite was enlarged from 2.53 nm to 3.01 nm. Acid red (AR) was used as a model pollutant to study the adsorption activity of the composites. The results showed that the adsorption process is dependent on pH, contact time, initial AR concentration and temperature. The maximal AR uptake by chitosan/rectorite composites was 95.82 mg g−1 in the test. Equilibrium isotherms showed a very good fit with the Langmuir isotherm equation for the monolayer adsorption process (R2 = 0.9646). Kinetics parameters of the adsorption process indicated that it followed the pseudo-second order equation, and the maximum sorption capacity calculated from the pseudo-second-order rate equation was 100.50 mg g−1 which was close to the experimental value. Adsorption thermodynamics study indicated the spontaneous nature and endothermic of the adsorption process.

 Please wait while we load your content...
Please wait while we load your content...