Retracted Article: A highly concise and practical route to clavaminols, sphinganine and (+)-spisulosine via indium mediated allylation of α-hydrazino aldehyde and a theoretical insight into the stereochemical aspects of the reaction†
Abstract
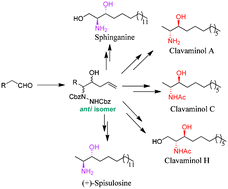
A conceptually different approach has been employed for the synthesis of 1,2-amino alcohols by proline-catalyzed α-amination of

 Please wait while we load your content...
Please wait while we load your content...