Recent advances in the Cu(i)-catalyzed azide–alkyne cycloaddition: focus on functionally substituted azides and alkynes
Abstract
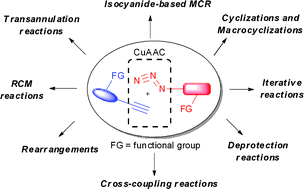
First described by the groups of Sharpless (Rostovtsev et al., Angew. Chem., Int. Ed., 2002, 41, 2596) and Meldal (Tornøe et al., J. Org. Chem., 2002, 67, 3057) in 2002, Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) became one of the most effective and reliable synthetic tools in diverse areas of modern chemistry, such as organic, medicinal and polymer chemistry, material science and chemical biology. The advantages of this reaction are high selectivity, simple reaction conditions, wide scope and excellent functional group tolerance. This efficient transformation can be used for the synthesis of various 1,2,3-triazoles. On the other hand, CuAAC can be efficiently used for bioconjugation of two units bearing alkyne and azide functions via the triazole moiety. Moreover, the use of azides and alkynes bearing orthogonal functional groups in this reaction opens broad opportunities for the synthesis of valuable polyfunctionalized triazole-containg molecules. Thus, the initial discussion is focused on synthetic applications of functionally substituted azides and terminal alkynes in the CuAAC, combined with other transformations. The prominent part of this review is devoted to an application of these bi (or tri)-functional reagents for the design, synthesis or labeling of biologically active molecules.
- This article is part of the themed collection: Organic chemistry collection

 Please wait while we load your content...
Please wait while we load your content...