Role of conformational properties on the transannular Diels–Alder reactivity of macrocyclic trienes with varying linker lengths†
Abstract
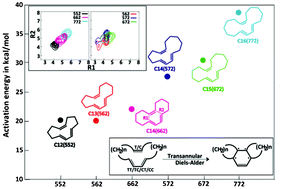
The effect of the linker length (–CH2–) connecting the diene and the dienophile in macrocyclic trienes on their transannular Diels–Alder (TADA) reactivity has been investigated using density functional theory (DFT) calculations. The relationship between the conformational properties of these reactants and their reaction energy barriers was examined and a quantitative relationship has been obtained. The transition state energy barriers were found to increase with an increase in the linker length, which is in contrast to the expected trend. The conformational preferences of the triene reactants were studied using replica exchange molecular dynamics (REMD) simulations. These calculations reveal that longer linkers lead to a decreased probability of the occurrence of conformations with the diene and dienophile parts of the system vicinal to each other, and thus lower reactivity of systems with long linkers. Excellent correlations between the transition state energies and the cumulative probabilities corresponding to a short distance between the reactive sites, along with the ability of the diene to exist in the s-cisoid form, were observed.

 Please wait while we load your content...
Please wait while we load your content...