Biphenyl dioxygenase-catalysed cis-dihydroxylation of tricyclic azaarenes: chemoenzymatic synthesis of arene oxide metabolites and furoquinoline alkaloids†
Abstract
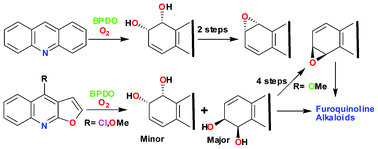
Biotransformation of acridine, dictamnine and 4-chlorofuro[2,3-b]quinolone, using whole cells of Sphingomonas yanoikuyae B8/36, yielded five enantiopure cyclic cis-dihydrodiols, from biphenyl dioxygenase-catalysed dihydroxylation of the carbocyclic rings. cis-Dihydroxylation of the furan ring in dictamnine and 4-chlorofuro[2,3-b]quinoline, followed by ring opening and reduction, yielded two exocyclic diols. The structures and absolute configurations of metabolites have been determined by spectroscopy and stereochemical correlation methods. Enantiopure arene oxide metabolites of acridine and dictamnine have been synthesised, from the corresponding cis-dihydrodiols. The achiral furoquinoline alkaloids robustine, γ-fagarine, haplopine, isohaplopine-3,3′-dimethylallylether and pteleine have been obtained, from either cis-dihydrodiol, catechol or arene oxide metabolites of dictamnine.

 Please wait while we load your content...
Please wait while we load your content...