Trisubstitution of pyridine through sequential and regioselective palladium cross-coupling reactions affording analogs of known GPR54 antagonists†
Abstract
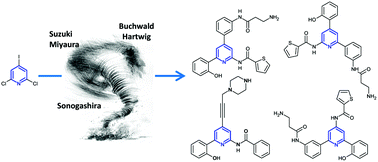
Because of their large spectrum of applications, poly-functionalized pyridines remain an attractive challenge in modern organic chemistry. We describe the poly-functionalization of halopyridines through a series of sequential and regioselective palladium-catalyzed cross-coupling reactions (Suzuki–Miyaura, Sonogashira and Buchwald–Hartwig reactions). This strategy was applied to the synthesis of several analogs of single non-peptidic known GPR54 antagonists.

 Please wait while we load your content...
Please wait while we load your content...