A soluble bispentacenequinone precursor for creation of directly 6,6′-linked bispentacenes and a tetracyanobipentacenequinodimethane†
Abstract
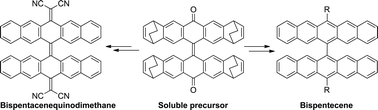
We have synthesized 13,13′-(3,5-bis(trifluoromethyl)phenyl)-6,6′-bipentacene from a soluble bispentacenequinone precursor. Bispentacene takes orthogonal conformation in the solid state and exhibits four reversible redox potentials. In addition, a tetracyanobipentacenequinodimethane was obtained for the first time from the pure bispentacenequinone.

 Please wait while we load your content...
Please wait while we load your content...