Inducing chirality on ZnS nanoparticles for asymmetric aldol condensation reactions†
Abstract
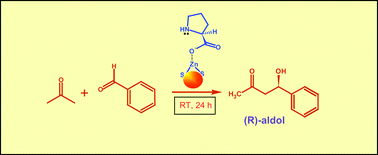
L-Proline immobilized ZnS nanoparticles (NPs) were synthesized by a simple wet chemical method and characterized by XRD. The as-synthesized NPs were used as a catalyst for the direct asymmetric aldol reaction of several aldehydes with acetone to achieve chiral β-hydroxy carbonyl compounds in good yields and enantioselectivity at room temperature. The peculiarity of our strategy is that the reaction is carried out at room temperature and does not involve any co-solvent for solubility purposes. The selectivity of the developed heterogeneous catalyst leads to only (R)-β-hydroxy carbonyl compounds and restricts the reaction at the aldolization stage only, without any formation of dehydrated α,β-unsaturated product. The modified reaction mechanism, showing the involvement of surface Zn+2 ions, is proposed. The catalyst was recovered and reused several times without any significant lost in activity. This opens new avenues for surface engineering leading to green catalytic processes.

 Please wait while we load your content...
Please wait while we load your content...