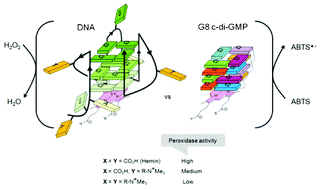
G-Quadruplex forming structures continue to be utilized for many biotechnological applications. Many parallel and mixed topology G-quadruplexes enhance the catalytic proficiency of hemin and there is an increasing trend to use these as non-protein-based peroxidase. Until now, the most proficient of nucleotide-based peroxidases have been oligonucleotides (DNA/RNA) that contain two or more G-tetrad planes and loop sequences. Herein we present a new “loopless” small molecule-based peroxidase, comprised of bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) octamer, capable of enhancing hemin peroxidation to the same extent as other well-known DNA peroxidases. Secondly, we reveal that the carboxylate moieties of hemin, the co-factor for nucleic acid-based catalysts, are important for catalysis because the replacement of the anionic carboxylate groups of hemin with ammonium moieties results in a decrease in peroxidation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...