Significant changes in pKa between bulk aqueous solution and surface immobilized species: ortho-hydroquinones
Abstract
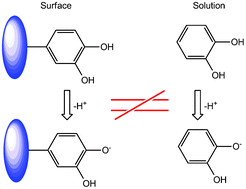
The pKa values of two ortho-hydroquinone H2Q, systems immobilized at a carbon electrode are investigated using voltammetric methods and compared with those of the corresponding molecules in bulk aqueous solution. A large and significant increase of both pKa values for the successive equilibria H2Q ⇆ HQ− + H+ ⇆ Q2− + 2H+ are observed, which highlight the fact that very significant differences of thermodynamic and chemical behaviour exist between molecules in bulk solution and immobilized at interfaces, both in general and in the case of

 Please wait while we load your content...
Please wait while we load your content...