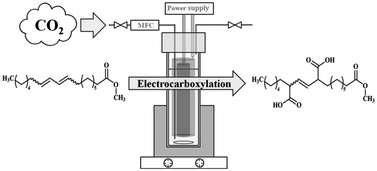
Carbon dioxide was electrochemically incorporated in internal conjugated dienes and the process was optimized to achieve satisfactory yields (>70%) even for less reactive substrates. Reactions were performed galvanostatically in an undivided cell at room temperature with a magnesium or aluminium sacrificial anode. Using an optimized electrosynthetic method for the dicarboxylation of 1,3-cyclohexadiene (optimal electrode material, CO2 pressure, amount of charge), the effect of molecular configuration and alkyl substitution on the reactivity of conjugated double bonds towards carboxylation was studied. Use of a bubble reactor at atmospheric pressure instead of a higher pressure reactor, and lowering of the current density made it possible to effectively perform the double carboxylation of internal conjugated double bonds in open chains. Conjugated linoleic acid methyl esters were used in this reaction for the first time and by searching for the optimal reaction conditions (solvent, supporting electrolyte, reactant concentration, amount of charge, current density) yields approaching 80% of the corresponding fatty triacid product could be obtained, at current efficiencies over 50%.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...