Translocation, transfer, and in vivo safety evaluation of engineered nanomaterials in the non-mammalian alternative toxicity assay model of nematode Caenorhabditis elegans†
Abstract
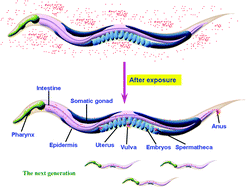
Translocation, transfer, and safety evaluation of engineered nanomaterials in non-mammalian alternative assay model of Caenorhabditis elegans are summarized and discussed. Due to the extensive applications of engineered nanomaterials (ENMs) in industry, agriculture, medicine and public health, environmental exposure of ENMs to human and environmental organisms is inevitable, therefore studies on translocation, transfer, and toxicology of ENMs receive wide interest. Modelling the nematode C. elegans, a widely recognized non-mammalian alternative toxicity assay system has been proven to be valuable in environmental safety evaluation, toxicological study, and examination of translocation and transfer of toxicants. In this review, we summarize and discuss the values and contributions of C. elegans in studies on environmental safety evaluation, toxicology, and translocation and transfer of ENMs. We introduced acute and chronic toxicity assay systems and discussed environmental safety assessment of specific ENMs at predicted environmental relevant concentrations, and influences of chemical properties of ENMs, exposure routes, developmental, genetic or physiological state, and environmental factors on nanotoxicity formation in nematodes. We then discussed the toxicological mechanisms of ENMs mainly with respect to roles of oxidative stress in normal and stress conditions and signaling pathways, reproductive toxicology, neurotoxicology, and translocation, distribution, transfer and metabolism of ENMs in C. elegans. Ways to eliminate or reduce the toxicities of specific ENMs by chemical modification with surface designs were further discussed. Finally, four important challenges have been raised and discussed by surrounding the aspects of environmental safety assessment, toxicological mechanisms, and designs of new safe ENMs.

 Please wait while we load your content...
Please wait while we load your content...