Oxidation of benzyl alcohols by semi-stoichiometric amounts of cobalt-doped birnessite-type layered MnO2 under oxygen atmosphere†
Abstract
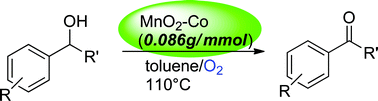
Semi-stoichiometric oxidation of benzylic alcohols to benzaldehydes was readily achieved in heated toluene in the presence of cobalt-doped birnessite MnO2 under oxygen atmosphere. The oxidation took place selectively for benzylic alcohols, while allylic alcohols were oxidized slowly. No oxidation occurred for usual primary and secondary alcohols. Oxygen atmosphere was important to perform effective oxidation; i.e. the oxidation progressed much slower in nitrogen atmosphere. Cobalt-doped birnessite was the best catalyst for the oxidation, in which the amount of birnessite was reduced to 0.7 equivalent of alcohol, resulting in yields of aldehydes greater than 80%. The present method provides a useful green oxidation for benzylic alcohols.

 Please wait while we load your content...
Please wait while we load your content...