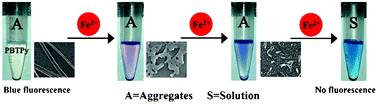
Iron is known to be indispensable for life since its levels are related to biochemical, pharmacological and toxicological functions in the organisms. Herein, we demonstrate a very simple visual detection method of iron(II) cations (Fe2+) with high specificity based on its etching effects on the blue fluorescent ribbon aggregates of 4′,4′′′′-(1,4-phenylene) bis(2,2′:6′,2′′-terpyridine) (PBTPy). PBTPy, a terpyridine attached molecule, is observed to form blue fluorescent ribbon aggregates in a pH-modulated solvent of DMSO/H2O. Owing to the complexing of two terpyridine functional groups with Fe2+, the aggregates of PBTPy are gradually “etched” by Fe2+, giving new absorption bands at around 576 nm, 582 nm and 610 nm with a color change from purple to blue and then to cyan, depending on the content of Fe2+. This response is highly specific for Fe2+ over many other metal ions, including Fe3+, and thus successfully applied to the detection of Fe2+ released from the ferritin.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...