On the thermodynamic phase behavior of poly(N-vinylcaprolactam) solution in the presence of different ionic liquids†
Abstract
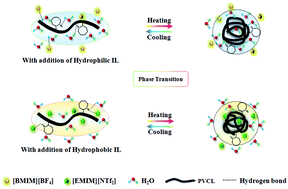
The influence of different Ionic Liquids (ILs) on the phase transition behavior of poly(N-vinylcaprolactam) (PVCL) solution is investigated by differential scanning calorimetry (DSC) and turbidity measurements, in combination with FT-IR spectroscopy. The DSC and turbidity results reveal that hydrophilic ILs at a concentration of 0.25 mol L−1 only slightly change the transition temperature. The LCST experiences an increase while the phase separation behavior disappears gradually as the concentration of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) increases from 0 to 1.0 mol L−1. In contrast, the addition of hydrophobic ILs, especially 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide ([EMIM][NTf2]), even at a low concentration, greatly raises the LCST, indicating the possibility of hydrogen bonding between [EMIM][NTf2] and PVCL. Finally, temperature-dependent FTIR spectra are employed to elucidate the dynamic mechanism of the different influences on the phase transition of PVCL. The hydrophilic ILs can change the state of the PVCL chains indirectly by varying their interaction with water molecules. For comparison, the formation of hydrogen bonds between the unsaturated C–H of [EMIM][NTf2] and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of PVCL results in a higher temperature and more energy to complete the phase transition. However, with the continual increasing of the concentration, extra [EMIM][NTf2] can not be well dispersed in water due to its hydrophobicity, so the LCST undergoes a decrease and finally reaches an equilibrium. Additionally, hydrophilic ILs tend to distribute in the solution, while hydrophobic ILs, especially [EMIM][NTf2], are prone to be wrapped in polymer chains of the aggregates after phase separation. Furthermore, both types of IL experience the process of hydrogen bond disruption and chain aggregation.
O of PVCL results in a higher temperature and more energy to complete the phase transition. However, with the continual increasing of the concentration, extra [EMIM][NTf2] can not be well dispersed in water due to its hydrophobicity, so the LCST undergoes a decrease and finally reaches an equilibrium. Additionally, hydrophilic ILs tend to distribute in the solution, while hydrophobic ILs, especially [EMIM][NTf2], are prone to be wrapped in polymer chains of the aggregates after phase separation. Furthermore, both types of IL experience the process of hydrogen bond disruption and chain aggregation.

 Please wait while we load your content...
Please wait while we load your content...