An effect on the side chain position of D–π–A-type conjugated polymers with sp2-hybridized orbitals for organic photovoltaics†
Abstract
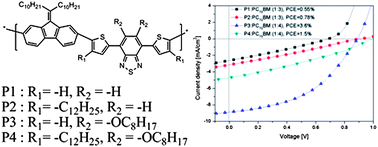
A D–π–A-type poly[alkylidenefluorene-alt-di-2-thienyl-2,1,3-benzothiadiazole] (P1) was synthesized via Suzuki coupling reaction. Based on the positions of the spacer (π) and acceptor (A) in the polymers, a dodecyl chain (P2) and an octyloxy chain (P3), respectively, were introduced. Both chains were introduced to the spacer and acceptor of P4. The obtained polymers (P2–P4) were soluble in organic solvents such as chlorobenzene, THF and o-dichlorobenzene at room temperature. Also, the introduction of a dodecyl chain to the spacer reduced the energy of the highest occupied molecular orbital (HOMO) level (−5.5 to −5.56 eV) but increased the tilt angle (45.4–53.0°), which had prevented the main chain from π–π stacking. The orientation of the obtained polymers in thin films was confirmed by XRD measurement. P3 with only an octyloxy chain showed a face-on-rich structure with a π–π stacking distance of 3.7 Å compared to other polymers. The polymer solar cells were fabricated through a solution process, and showed a power conversion efficiency (PCE) of 3.6%, with a short-circuit current density (Jsc) of 8.9 mA cm−2, an open-circuit voltage (Voc) of 0.88 V, and a fill factor (FF) of 45.7%. With the introduction of poly[9,9-bis(6′-(diethanolamino)hexyl)fluorene] (PFN-OH), a PCE of 3.9% was confirmed.

 Please wait while we load your content...
Please wait while we load your content...