Ring-opening polymerization of racemic β-butyrolactone promoted by rare earth trisborohydride complexes towards a PHB-diol: an experimental and DFT study†
Abstract
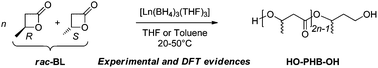
The ring-opening polymerization (ROP) of racemic β-butyrolactone (BL), catalyzed by the homoleptic lanthanide trisborohydride complexes, [Ln(BH4)3(THF)3] with Ln = La, Nd, and Sm, is reported, together with a DFT study of the polymerization mechanism. Well-defined atactic α,ω-dihydroxytelechelic poly(3-hydroxybutyrate)s (PHBs), PHB diols, are thus synthesized (Mn up to 8000 g mol−1, 1.02 ≤ Đ ≤ 1.10) as evidenced both experimentally and computationally. DFT investigations also emphasize the lack of stereoselectivity of the catalyst although a high activity is energetically evidenced.

 Please wait while we load your content...
Please wait while we load your content...