Smart heparin-based bioconjugates synthesized by a combination of ATRP and click chemistry†
Abstract
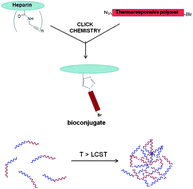
This article describes the synthesis of novel well defined polysaccharide–polymer bioconjugates. Alkyne-containing bioconjugates were prepared using Cu(I) catalyzed azide–alkyne [3 + 2] dipolar cycloaddition, i.e. ‘click’ chemistry between an alkyne functionalized low molecular weight heparin (LMWH) and α-azide functionalized stimuli-responsive copolymers synthesized by atom transfer radical polymerization using Activators Generated by Electron Transfer (AGET). The alkyne functionality was incorporated into the heparin by conducting a high yield amidation of the polysaccharide using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride. The structure and composition of the novel bioconjugates were confirmed by FTIR, NMR and thermogravimetric analysis (TGA).

 Please wait while we load your content...
Please wait while we load your content...